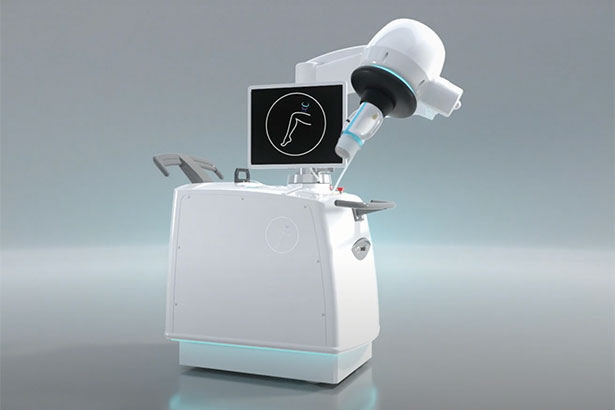
June 1, 2022 — As part of a U.S. Food and Drug Administration (FDA) approved, investigator-initiated clinical trial, Englewood Health is offering a new treatment for varicose veins that utilizes an emerging technology: Sonovein® echotherapy. This no-incision, no-scar treatment is designed to provide patients relief from their varicose vein symptoms and a quick return to normal activities following the procedure.
On May 6 and 7, Englewood Health entered the first four patients to receive this novel therapy into the trial. These patients also mark the first in the U.S. to undergo this treatment for varicose veins. In each case, the Sonovein treatment was administered without the need for any kind of anesthesia or infiltration.
Steve Elias, MD, director of the Center for Vein Disease at Englewood Health explained, “We appreciate the opportunity to be the first and only hospital to trial this technology for this indication. But more importantly, we really appreciate our patients for participating and helping to improve vein care for all.”
Sonovein represents the only completely noninvasive varicose vein treatment option using echotherapy to treat damaged veins from outside the body. During echotherapy, high-intensity focused ultrasound (HIFU) waves target veins and generate thermal energy. As thermal energy is delivered to the veins, they shrink and ultimately seal closed.
At least three out of every 10 people experience varicose veins in their lifetimes. In a healthy leg vein, the surrounding muscles, combined with the vein walls and valves, work together to ensure that blood is transported against gravity from the legs back towards the heart.
If the vein valves no longer function properly—or the elasticity of the vascular walls weaken—a blood build-up can occur in the legs. This enlarges the vein walls, causing varicose veins to develop and the possible onset of symptoms like itchy, swollen, or heavy-feeling legs. If left untreated, such symptoms can worsen over time, resulting in leg ulcers and other complications.
For those wondering whether they indeed have varicose veins, a consultation with a vein specialist will provide clarity as to whether their veins are functioning properly or if treatment might be necessary.
About the Trial
This is a single-center study with a planned accrual of 20 patients with diagnosed symptomatic primary great saphenous vein (GSV) insufficiency. Interested patients will provide informed consent and be screened for participation.
If eligible, participants will be scheduled for a one-day Sonovein treatment, which consists of:
- Pre-treatment ultrasonography
- Positioning
- Planning
- Generation of HIFU pulses in the segments chosen for treatment
- Post-treatment visualization
At follow-up visits scheduled for one week after treatment and 3 months after treatment, changes in veins and flow characteristics will be evaluated by ultrasound and physical exam.
There is no cost to patients who qualify for participation in the trial. If interested in participating, you can reach Dr. Elias at the Center for Vein Disease at Englewood Health, 201-894-3252.

